Abstract
Introduction
Multiple myeloma (MM) is a plasma cell malignancy, with a range of clinical features including hypercalcaemia, renal insuficiency, anaemia and bone lesions1 (CRAB). MM progression often involves multiple rounds of remissions after treatment followed by subsequent relapses2. Although MM accounts for only a small percentage of all cancer types, the costs associated with treating and managing it are among the highest3.
RRMM population is heterogeneous, their characteristics depend on the number and type of treatment used, and the type of relapse3.
Selection of treatment for MM patients is challenging due to increased age and the often associated comorbid conditions4. RRMM patients are more symptomatic, vulnerable to adverse events and, more likely to incur a dose reduction or early discontinuation of therapy. Balancing expected efficacy of therapy vs potential toxicities, health care resources and impact on patients' Health Related Quality of Life (HRQOL) is therefore critical in this patient population5.
CharisMMa study objective (NCT03188536) is to understand and provide an accurate epidemiological and societal characterization of RRMM patients. Hematologist's choice of therapeutic strategy is crucial, and must be made in consideration of the patient as a whole, and not only in terms of their disease.
Methods
CharisMMa is an observational, cross-sectional, multicentre study, involving 30 public hospitals around Spain, which include MM patients that require treatment at any relapse of the disease. An interim analysis has been conducted evaluating socio-demographic characteristics and burden of the disease in the 169 patients with relapse/refractory MM enrolled up to June 2018 (expected n=350). On average, information was collected 2.15 (SD: 1.76) months after the last relapse.
Results
The median age in the moment of the study visit, was 69.0 (57.0, 75.0) years, being 55.2% male. 71.8% of them lived in urban areas and 88% with their families. Most of them were retired (64.2%) and non-dependent (73.1%). Patients included have been treated with a median of 2.0 (1.0, 3.0) previous lines. Approximately half of them (56.7%) had received stem cell transplant (97.8% autologous). The stage ISS was I (36.3%), II (35.6%) and III (28.1%) and 74.0% presented CRAB symptoms, being bone lesions (65.6%) the most frequent ones. 85.4% were considered intermediate or high risk patients presenting extramedullary plasmocytomas in 16.5% of the cases. 67.5% of the patients suffered from any comorbidity, being the most frequent one cardiovascular (48.1%), diabetes (23.1%) and neuropathy (22.1%).
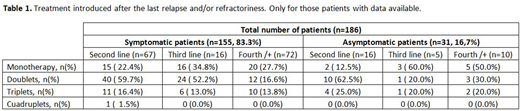
Symptomatic patients are treated mainly with doublets both in second (59.7%) and third/+ lines while those asymptomatic ones with no CRAB symptoms at relapse are treated with doublets in second but with monotherapy (62.5%) in third/+ lines (Table 1).
In terms of societal perspective, on average, patients are living 23.9 (40.4) km far from their hospital and they attend 5.4 (5.0) times per month in order to visit the haematologist being 3.8 (2.7) times treatment related. In most of the visits (90.3%) patients attended accompanied by a caregiver who is occupationally active (57.4%). Only during the last relapse patients have been admitted 1.5 (1.0) times to the hospital having spent 15.2 (15.5) days until discharge. The patients are also visiting other specialist during the last relapse due to the disease as primary health care professionals (21.3%), psychologists (4.7%) and others (33.1%).
Conclusions
The heterogeneity in the MM patient´s characteristics is a major factor that should be considered during the management and the evolution of the disease and it has a direct impact in the resources needed. An individual approach including patients and disease characteristics should be considered in order to maximize clinical outcomes and guarantee an appropriate use of the resources in order to maximize patient´s benefit and reduce indirect costs of the disease.
References
Palumbo, K. Anderson. N. Engl. J. Med. 364 (2011)1046-1060.
National Cancer Institute. SEER Program. Cancer stat facts: myeloma. 2016. Available at https://seer.cancer.gov/statfacts/html/mulmy.html
Laubach J et al. Leukemia 2016; 30(5):1005-1017.
Sonneveld P, Broijl A. Haematologica 101(4):396-406.
Larocca A, Palumbo A. Blood 2015; 126 (19):2179-2185.
Ocio:BMS: Consultancy; Seattle Genetics: Consultancy; AbbVie: Consultancy; Pharmamar: Consultancy; Janssen: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Sanofi: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Mundipharma: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Array Pharmaceuticals: Research Funding. Rosinol:Janssen, Celgene, Amgen, Takeda: Honoraria. Grande:Takeda: Employment. Ruiz-Zorrilla:Takeda: Employment. Fernandez:Takeda: Employment. Montoto:Takeda: Employment.
Author notes
Asterisk with author names denotes non-ASH members.